The following questions are based on material presented in EMS 11 (part A; Kinemages 2-5) and material in chapter 11 of your textbook.
1) (K2)
a) Examination of the backbone sequences for the portion of rubredoxin which supplies the ligands to the iron ion in the protein shows that two stretches of 4 amino acids each (which can be short-handed as Cys-X-X-Cys) act to tether the metal ion to the protein. What are the two sequences for the Rubredoxin shown?
b) Using the measures command estimate the bond angles and lenths for the Fe-S bonds in rubredoxin.
c) What is the approximate geometry of the Fe-S cluster in rubredoxin?
2) (K3)
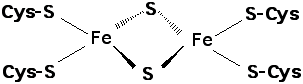
a) A schematic for the [2Fe-2S]
cluster from ferridoxin is shown below:

i) Using Measures supply the bond distances for each Fe-S bond shown.
ii) Measure the bond angles around each of the Fe ions. What is the approximate
coordination geometry for each of the Fe ions?
iii) Four of the eight atoms shown fall in a plane. Circle the planar portion of the
structure given above.
b) The [2Fe-2S] cluster is sometimes notated as Fe2S2(Scys)4 to explicitly show the involvement of the cys S. Assuming that each inorganic (bridging) S carries a formal charge of -2 and that each cysteine sulfur carries a formal charge of -1, calculate the formal charge in the fully oxidized complex given that both Fe in the oxidized complex are Fe+3.
c) The reduced form of the [2Fe-2S] cluster has one Fe+2 and one Fe+3 ion. What is the formal charge on the entire complex in the reduced form?
d) When calculating the formal charge
on the cluster itself, it is possible to ignore the charge from the Cys
S (supplied by the protein) and simply calculate the charge on the cluster
using the Fe and inorganic S. The resultant cluster is notated [2Fe-2S]+nwhere
n is the formal charge on the cluster. Using this notation designate the
charge on the oxidized and reduced clusters.
3) (K4)
a) Examine the [4Fe-4S] cluster from the HIPIP protein. How are the Fe ions attached to the protein?
b) A [4Fe-4S] cluster is often called a cubane given its similarities to a cube. Measure the angles at which each Fe and sulfur atom is bound to the cube. Is the Fe-S cluster shown a perfect cube?
c) Examine the coordination geometry of each Fe ion. What is it?
d) Calculate the formal charge on the oxidized form of the [4Fe-4S] cluster given that three of the Fe ions are Fe+3 and one is Fe+2.
4) (K5)
a) Unlike the heme in hemoglobin, the heme group in cyt c is covalently attached to the protein via cys residues. What sites on the heme ring are the cys residues attached to (propionic, vinyl, or methyl)? Draw a structure of the heme group in cyt c.
b) The Fe ion is coordinated to the protein via two amino acid residues. Which amino acid residues are they? How does this compare to the Fe ion coordination in myglobin?
c) The Fe ion is Fe+3 in the oxidized form and Fe+2 in the reduced form. Examine the differences in absorption spectra for the two oxidation states of the cyt c shown below. Assuming that you are interested in doing an experiment using cytochrome c+3 as an electron acceptor and using a spectrophotometer to measure the extent of reduction of cyt c, what would be the best wavelength to set the spectrophotometer at?
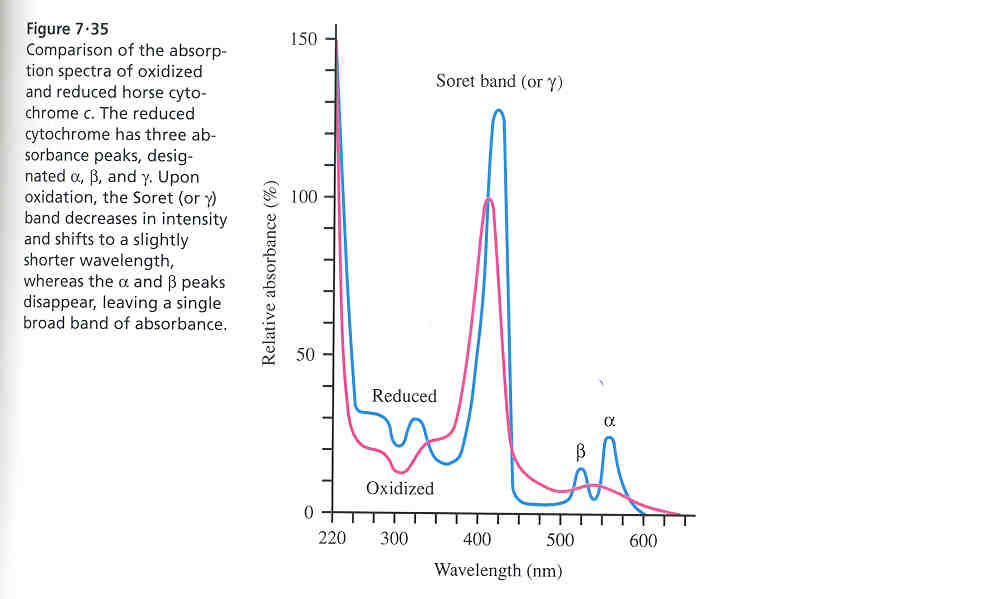
(Taken from Principles of Biochemistry by Horton et al., 2nd edition, Prentice Hall, Upper Saddle River, NJ)