The basic building block of the vast majority of rocks on Earth are minerals. Minerals are composed of atoms. In order to understand rocks, we must first have an understanding of minerals. In order to understand minerals we must have some basic understanding of atoms - what they are and how they interact with one another to form minerals.
Definition of a Mineral:
1. It must be Naturally formed it forms in nature on its own (without the aid of humans)
2. It must be a Solid
3. It must be inorganic i.e. not the product of biologic activity or processes (i.e. shells, tree sap or the like)
4. With a definite chemical composition (every time we see the same mineral it has the same chemical composition that can be expressed by a chemical formula).
5. It must have a characteristic crystalline structure (atoms are arranged within the mineral in a specific ordered manner).
Examples
Glass - can be naturally formed (volcanic glass called obsidian), is a solid, its chemical composition, however, is not always the same, and it does not have a crystalline structure. Thus, glass is not a mineral.
Ice - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula H2O, and does have a definite crystalline structure when solid. Thus, ice is a mineral, but liquid water is not (since it is not solid).
Halite (salt) - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula NaCl, and does have a definite crystalline structure. Thus halite is a mineral.
Atoms
Atoms are the smallest divisible components of matter that have the same properties (e.g. size, shape, mass) which differ from the properties of other elements. They are the fundamental units of each mineral's crystal structure. Each atom consists of a tiny nucleus that contains positively-charged protons and electrically neutral neutrons. This nucleus is surrounded by one or more shells of negatively-charged electrons. A neutral atom has the same number of protons and electrons.
An atom is composed of three different particles:
Protons -- positively charged, reside in the center of the atom called the nucleus
Electrons -- negatively charged, orbit in a cloud around nucleus
Neutrons -- no charge, reside in the nucleus.
Number of protons = Number of electrons.
Number of protons = atomic number.
Number of protons + Number of neutrons = atomic weight.
Isotopes are atoms of the same element with differing numbers of neutrons. i.e. the number of neutrons may vary within atoms of the same element. Some isotopes are unstable which results in radioactivity.
Example:
K (potassium) has 19 protons. Every atom of K has 19 protons. Atomic number of K = 19. Some atoms of K have 20 neutrons, others have 21, and others have 22. Thus atomic weight of K can be 39, 40, or 41. 40K is radioactive and decays to 40Ar and 40Ca.
Structure of Atoms:
|
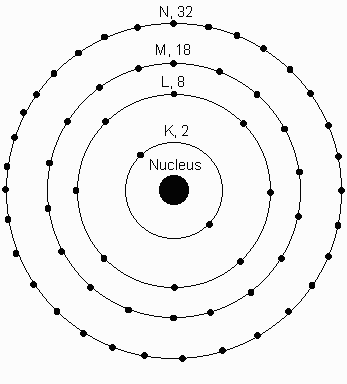
Electrons orbit around the nucleus in different shells, labeled from the innermost shell as K, L, M, N, etc. Each shell can have a certain number of electrons. The K-shell can have 2 Electrons, the L-shell, 8, the M-shell 18, N-shell 32.
For simplicity you can think of these shells as amusement park rides, each with a certain number of seats available on the ride. For example the K ride has 2 seats, the L ride has 8 seats etc.
A stable electronic configuration for an atom is one with a completely filled outer shell. Thus, atoms often loose, gain or share electrons to obtain stable configuration. Noble gases have completely filled outer shells, so they are stable and under normal circumstances non-reactive (i.e. "inert"). Examples He, Ne, Ar, Kr, Xe, Rn.
|
|
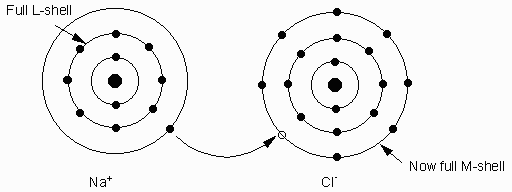
Others like Na and K loose an electron. This causes the charge balance to become unequal. In fact these atoms become positively ( + ) charged atoms called ions. Positively charged atoms are called cations. Elements like F, Cl, O gain electrons to become negatively ( - ) charged. Negatively charged ions are called anions.
The drive to attain a stable electronic configuration in the outermost shell along with the fact that this sometimes produces oppositely charged ions, results in the binding of atoms together. When atoms become attached to one another, we say that they are bonded together.
|
|
Types of bonding:
Ionic bonding occurs when one or more electrons are donated by one atom and received by another, resulting in charge imbalances in each of the atoms - turing them into ions. The atoms are bonded together by the force of attraction between ions of opposite charge. Ionic bonds are the most important in 90% of all minerals. Each ion is surrounded by ions of opposite charge.
Example Na+1 and Cl-1. Bond to form NaCl (halite or salt).
Covalent bonding - Electrons are shared between two or more atoms so that each atom has a stable electronic configuration (completely filled outermost shell) part of the time. Pure covalent bonds are rarely the predominant type in minerals. In the diamond structure, a central carbon atom is surrounded by four other carbon atoms each of which shares an electron with the central carbon atom.
Both ionic and covalent bonds typically occur in the same compound (bonds are seldom 100% ionic or covalent in nature)
Example: H has one electron, needs to 2 to be stable. O has 6 electrons in its outer shell, needs 2 to be stable. So, 2 H atoms bond to 1 O to form H2O, with all atoms sharing electrons, and each atom having a stable electronic configuration 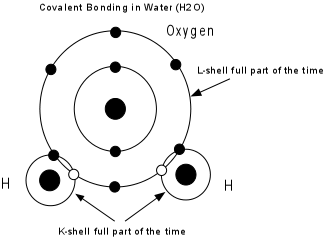
Metallic bonding -- is the most common type within native metals like copper and gold. Metallic bonding occurs when electrons can move freely from one ion to another. In materials that bond this way, electrons move freely from atom to atom and are constantly being shared. Materials bonded with metallic bonds are excellent conductors of electricity because the electrons can move freely through the material.
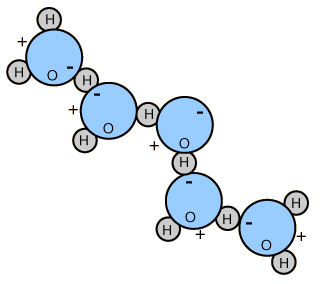
Van der Waals bonding - is a weak type of bond that does not share or transfer electrons. These bonds are caused by attraction between dipolar molecules; molecules with a charge imbalance created by their structure (permanent dipole) or created temporarily by the movement of electrons (temporary dipole).
|
For example - the electrons on a water molecule are locked into contact with the oxygen atom, leaving the exterior of the hydrogen atoms with a slight positive charge and the opposite side of the molecule with a slight negative charge. The slight charges will cause the water molecules to be attracted to each other. The same slight charges also occur in mineral structures.
This type of bounding usually results in a zone along which the material breaks easily (cleavage). A good example is graphite (see figure 3.11 in your text). Several different bond types can be present in a mineral, and these determine the physical properties of the mineral.
|
Crystal Structure
Packing of atoms in a crystal structure requires an orderly and repeated atomic arrangement. Such an orderly arrangement needs to fill space efficiently and keep a charge balance. Since the size of atoms depends largely on the number of electrons, atoms of different elements have different sizes.
Example of NaCl :
For each Na atom there is one Cl atom. Each Na is surrounded by Cl and each Cl is surrounded by Na. The charge on each Cl is -1 and the charge on each Na is +1 to give a charged balanced crystal.
|
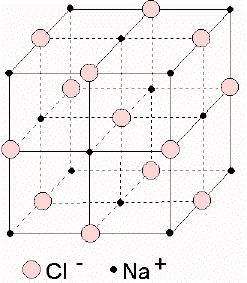
|
The structure of minerals is often seen in the shape of crystals.
Polymorphs are minerals with the same chemical composition but different crystal structures.
Ionic Substitution (Solid Solution)
Ionic substitution - (also called solid solution), occurs because some elements (ions) have the same size and charge, and can thus substitute for one another in a crystal structure.
Examples:
Olivine ranges from Fe2SiO4 to Mg2SiO4. Fe+2 and Mg+2 are about the same size, thus they can substitute for one another in the crystal structure and olivine thus can have a range of compositions expressed as the formula (Mg,Fe)2SiO4.
Alkali Feldspars: KAlSi3O8 (orthoclase) and NaAlSi3O8, (albite) K+1 can substitute for Na+1
Plagioclase Feldspars: NaAlSi3O8 (albite) and CaAl2Si2O8 (anorthite) NaSi+5 can substitutes for CaAl+5 (a complex solid solution).
Composition of Minerals
The variety of minerals we see depend on the chemical elements available to form them. In the Earth's crust the most abundant elements are as follows (actually these numbers will vary a bit depending on which book you look at - see page 8 in your text):
O, Oxygen 46.6% by weight
Si, Silicon 27.7%
Al, Aluminum 8.1%
Fe, Iron 5.0%
Ca, Calcium 3.6%
Na, Sodium 2.8%
K, Potassium 2.6%
Mg, Magnesium 2.1%
All others, 1.7%
Note that Carbon (one of the most abundant elements in the biosphere) is not among the top 8.
There are over 4,000 known minerals, but because of the limited abundance of elements present in the Earth's crust, only about 20 to 30 of these minerals are common. The most common minerals are those based on Si and O: the Silicates. Silicates are based on (SiO4)4- tetrahedron. Four oxygen atoms are covalently bonded to one silicon atom.
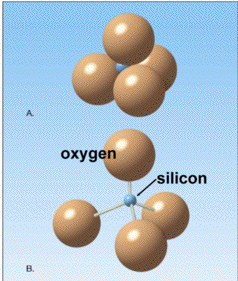
Properties of Minerals
Physical properties of minerals allow us to distinguish between minerals and thus identify them. Among the common properties used are:
- Habit - shape
- Color
- Streak (color of fine powder of the mineral)
- Luster -- metallic, vitreous, pearly, resinous (reflection of light)
- Cleavage (planes along which the mineral breaks easily)
- Specific Gravity (or Density [mass/volume])
- Hardness: based on Mohs hardness scale as follows:
- Talc
- Gypsum (fingernail)
- Calcite (penny)
- Fluorite
- Apatite (knife blade)
- Orthoclase (glass)
- Quartz
- Topaz
- Corundum
- Diamond
Back to GES101 homepage





